New Suprapubic Catheterisation Kit Alleviates Mortality And Complication Risks
Pioneering technology showcased at AUA offers clinicians a safer, more controlled technique
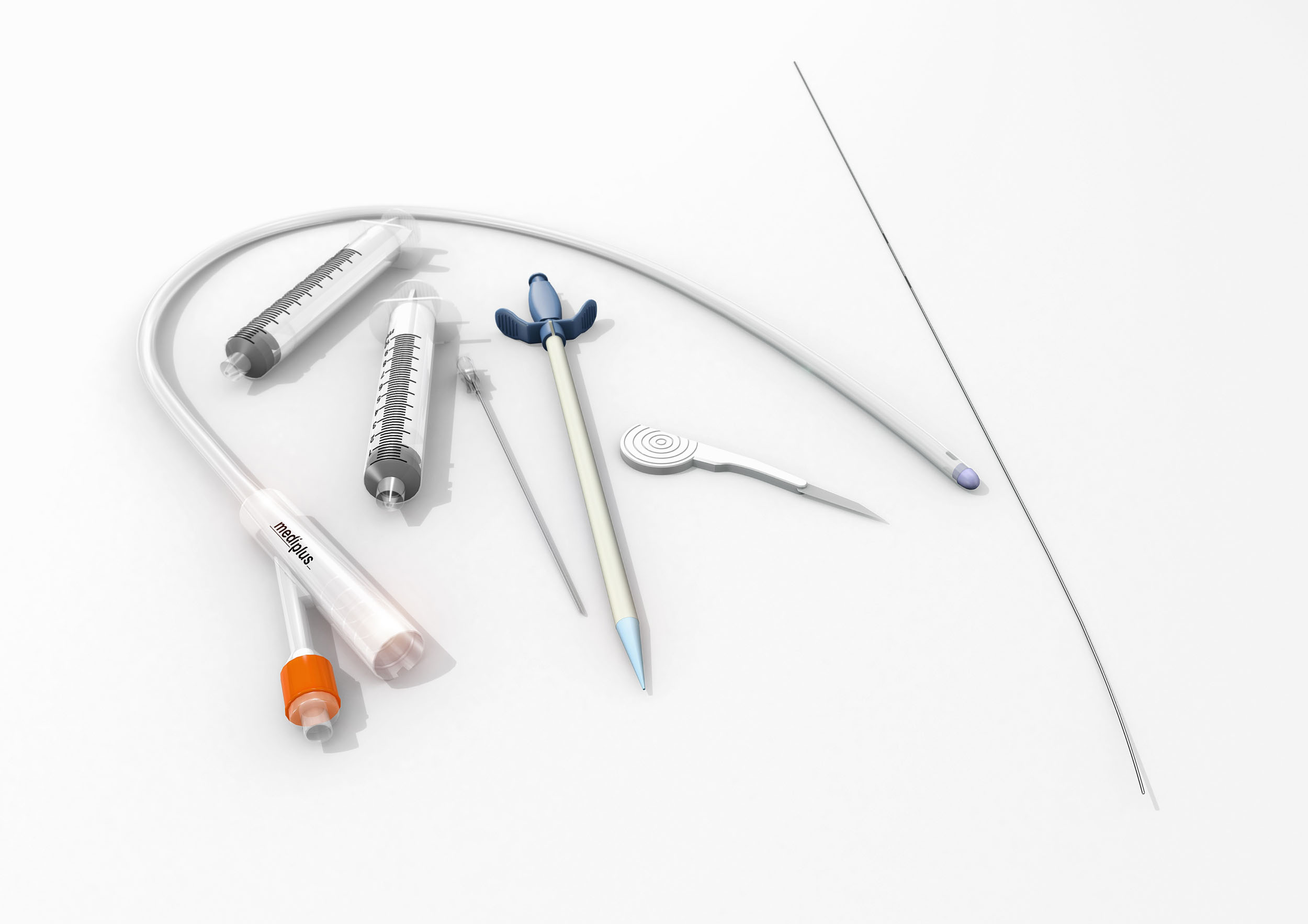
A new pioneering catheter insertion kit that allows clinicians to perform a safer introduction of a suprapubic Foley catheter will be unveiled at the American Urology Association (AUA).
Dubbed GPS for the bladder, the S-Cath™ System utilises the Seldinger technique to facilitate a one tract entry offering clinicians a more controlled, accurate and precise placement into the bladder.
The breakthrough technology decreases the risks associated with standard ‘blind’ methods which have 29%* risk of complications and 4.4% risk of mortality**.
It has also just been presented with a Queen's Award for Enterprise, under the innovation category, by Her Majesty Queen Elizabeth II for its technical and clinical excellence.
Typically, suprapubic catheterisation is performed in an operating theatre under general anesthetic with clinicians using a ‘blind’ percutaneous trocar puncture to gain entry into the bladder.
With S-Cath™ System, patients attend a 30-minute appointment slot in clinic, thus reducing treatment time from 2.3 days to 29 minutes. This offers hospitals huge savings in terms of money, time and resources.
The system also uses a patented system that locates the bladder with the safety guide wire; improving placement and guarantees insertion of the trocar along the anesthetised track.
This technique also offer a wide range of patient benefits such as minimal trauma and tissue damage as less pressure is needed to insert the dilator. It is also more comfortable and less stressful for the clinician to perform, as it provides them with a higher degree of control and accurate placement.
The S-Cath™ System is supplied in a standardised kit with all the necessary equipment together in one place, helping to save the clinician valuable time. It can be used on both men and women and can be performed by junior clinicians and nurses; freeing up consultants' time and enhancing out-of-hours services.
It has been used in the UK for six years and the UK National Institute for Clinical Excellence (NICE) and The British Association of Urological Surgeons (BAUS) have both published guidelines encouraging the adoption of S-Cath™ System into routine care.
Emma Gray, Managing Director, said: "The US health care model is moving to value-based care which requires adoption of new technology. S-Cath™ System is a breakthrough solution that can improve patient care and reduce the risk of harm to patients and providers, whilst simultaneously raising the standard of patient care and safety".
“Clinicians who have used the kit in the US say it offers them greater confidence in inserting the trocar into the bladder as the track has already been secured by the guide wire. Many are already describing it as a GPS for the bladder.”
Based in the UK, Mediplus have been supplying the healthcare sector with innovative patient centric products for 30 years. The company, who specialise in Urology and Anesthesia products, will work with a growing number of specialist distributors to launch the S-Cath™ System across the US.
To find out more visit Mediplus at the American Uro
CONTACT
James Urie
Mediplus Ltd
marketing@mediplus.co.uk
www.mediplus.co.uk
+44 1494 551200
Friday 12 May 2017 / file under Healthcare | Medical



